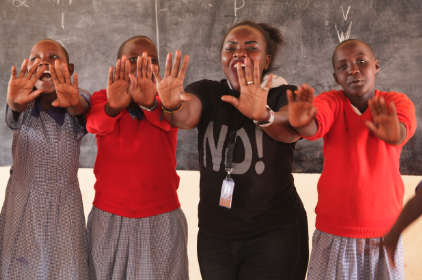By: Jocelyn Mackie, Co-CEO, and David Brook, Chief Strategy Officer, Grand Challenges Canada.
A recent New York Times article, Moderna and U.S. at Odds Over Vaccine Patent Rights, highlights an all-too-common challenge in the world of public-private partnerships for health research and innovation—who has the legal right to deploy the novel products and services that are developed, particularly during a global health crisis? Most of the time, this is simply a question of who gets to profit from what can be incredibly lucrative Intellectual Property or IP. In the case of the COVID-19 vaccine developed by Moderna and the U.S. government, however, this question has much deeper implications for the health and transmission of COVID in communities and countries around the globe and so, by extension, for all of us.
Unfortunately, ensuring access to life-saving pharmaceuticals and health products for underserved communities and countries is often an afterthought, or non-existent priority for governments when they fund health innovation. Instead, the emphasis is too often placed solely on supporting new research and commercializing the ideas of innovations that are produced. This is good for pharmaceutical companies but not so helpful in global health crises like the COVID pandemic. Relying on market forces, assumptions about co-invention, or the goodwill or moral compass of companies like Moderna was a grave mistake by the U.S.’ National Institutes of Health, or NIH, that cannot be repeated.
So how can public funders of research like the NIH, Canadian Institute of Health Research, or National Research Council Canada ensure that the products developed with their funding are readily and affordably available to address future global crises? This is an area where domestic research funders can learn some important lessons from global health innovation platforms like Grand Challenges Canada.
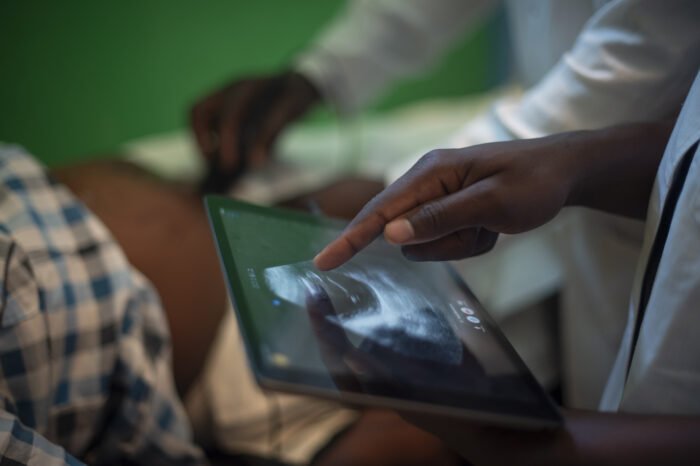
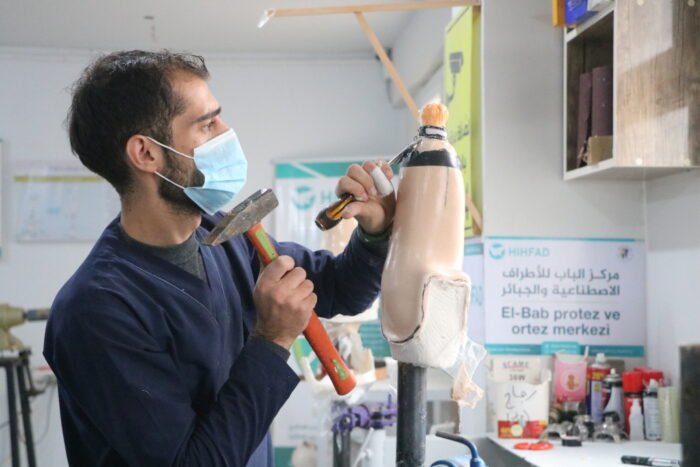
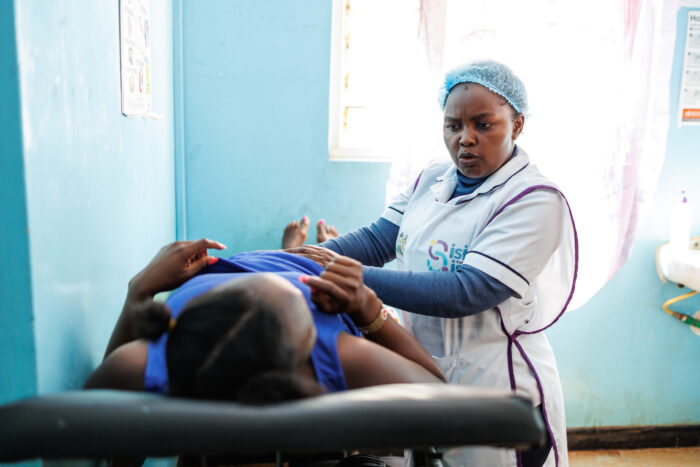
As a funder of global health innovation, issues of global access (ensuring that underserved populations have access to life-saving health products and services) are at the core of our work. Because of this, we build a commitment to global access at all stages of our funding processes, particularly as we support ideas that have achieved proof-of-concept and begin to go to scale. At the core of our approach to global access is a commitment that gives us the right to a non-exclusive license for all intellectual property (importantly, including its underlying/enabling intellectual property) that is developed with our funding. This license only comes into effect if the IP owner chooses not to make a resulting product available at a reasonable price in low- and middle-income countries.
Our approach allows companies to keep ownership of the intellectual property they create and to capitalize on it in high-income markets, to sell their product for profit in countries that can afford it. This allows for sustainable profit generation which enables companies to raise capital to build factories, source raw material, and pay employees, an important aspect of scaling innovations as Jacob S. Sherkow rightly points out. At the same time, our approach also ensures that there is an enforceable license in place that creates a strong incentive for inventors to also make their products available in underserved markets.
In relevant circumstances, we also add a worldwide license that kicks in if the World Health Organization (WHO) declares a Public Health Emergency of International Concern, such as COVID-19. In such cases, we can then also license the resulting product for accessible access worldwide. In the case of the Moderna vaccine, had the NIH required this form of license in exchange for its billions of dollars in funding, the U.S. government would have had more bargaining power to access the vaccine for use by high-income countries too, like Canada.
Moderna’s global rollout of its COVID vaccine would have looked very different if it had been subject to a strong and legally binding global access license, helping to prevent the current vaccine apartheid that is inhibiting the world from getting beyond the COVID-19 pandemic. All public funders of research need to learn from NIH’s failing and prioritize global access from the beginning of their funding partnerships and not as an afterthought once the work is concluded. Our ability to respond to future global crises depends on it.